Month: July 2025
All-ferroelectric implementation of reservoir computing
In the article “All-ferroelectric implementation of reservoir computing”, published in Nature Communications, Zhiwei Chen, Wenjie Li, Shuai Dong, Z. Hugh Fan, Yihong Chen, Xubing Lu, Min Zeng, Minghui Qin, Guofu Zhou, Xingsen Gao, and Jun-Ming Liu report a novel approach for implementing reservoir computing (RC) using a monolithic, fully ferroelectric hardware platform. This work is a result of multidisciplinary collaboration among experts in ferroelectric materials, neuromorphic device engineering, and condensed matter physics.
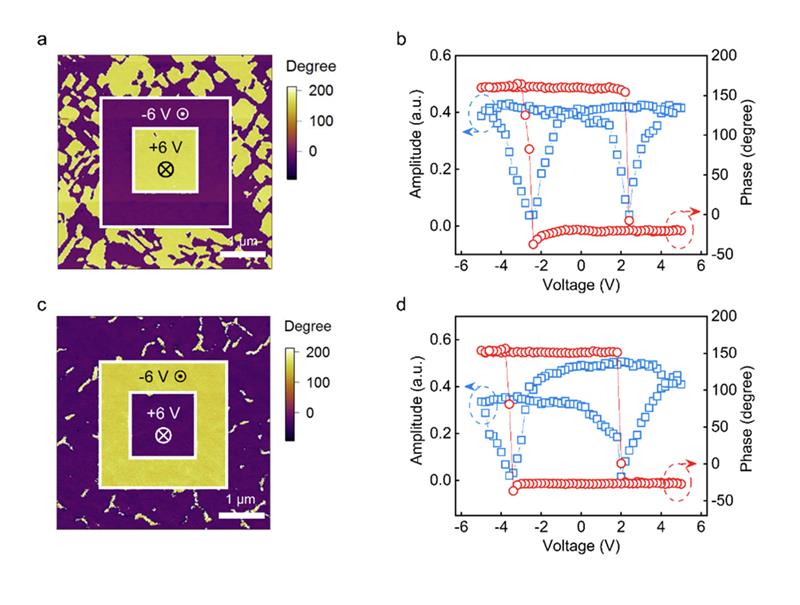
Reservoir computing is a recurrent neural network model that excels at processing spatiotemporal data, typically requiring complex and heterogeneous hardware. In this study, the authors demonstrate that a single material system—epitaxially grown Pt/BiFeO₃/SrRuO₃ ferroelectric thin films—can simultaneously implement both volatile and nonvolatile functionalities required for RC. This is achieved through precise imprint field (E_imp) engineering, which modifies the polarization dynamics within the ferroelectric layer.
Two types of ferroelectric diodes (FDs) are fabricated from the same stack:
• Volatile FDs, grown at a oxygen pressure of 19 Pa, possess a nonzero imprint field, resulting in spontaneous polarization back-switching after the removal of input pulses. This gives rise to short-term memory and fading dynamics, which are ideal for temporal feature transformation in the reservoir layer.
• Nonvolatile FDs, grown at a oxygen pressure of 15 Pa, with minimal imprint field, exhibit stable long-term potentiation/depression (LTP/LTD), making them well-suited for synaptic weight storage in the readout layer.
The all-ferroelectric RC system was benchmarked on several temporal processing tasks:
• Chaotic Hénon map prediction with a normalized root-mean-square error (NRMSE) of 0.017,
• Waveform classification (NRMSE ≈ 0.13),
• Noisy handwritten digit recognition (up to 91.7% accuracy), and
• Curvature discrimination (100% accuracy).
The devices showed remarkable endurance (>10⁶ cycles), retention (>30 days), low variability (~8% cycle-to-cycle), and extremely low power consumption (~11.8 µW for volatile, ~140 nW for nonvolatile). These results affirm the potential of ferroelectric devices for ultralow-power, scalable neuromorphic computing.
To support these findings, the study employed high-resolution scanning probe microscopy techniques. Specifically, NanoWorld Arrow™ EFM conductive AFM probes were used for piezoresponse force microscopy (PFM). These measurements were critical in confirming that volatility and nonvolatility were governed by tunable imprint fields within the BiFeO₃ layer.
The exceptional electrostatic sensitivity, sharp tip radius, and stable mechanical properties of NanoWorld Arrow™ EFM probes were indispensable in characterizing the field-induced polarization behavior and validating the dual-mode operational framework of the ferroelectric diodes.
This work presents a significant advance in neuromorphic hardware, showing that imprint-field engineering in ferroelectric systems enables the unification of dynamic and static memory functions within a single material system. The integration of volatile and nonvolatile functions into a coherent architecture—combined with robust nanoscale characterization—offers a promising path toward compact, energy-efficient RC platforms based entirely on functional oxides.
Citation:
Chen, Z., Li, W., Dong, S., Fan, Z. H., Chen, Y., Lu, X., Zeng, M., Qin, M., Zhou, G., Gao, X., & Liu, J.-M. (2023). All-ferroelectric implementation of reservoir computing. Nature Communications, 14, 3851. https://doi.org/10.1038/s41467-023-39371-y Read full article here
Deed – Attribution 4.0 International
– Creative Commons
Phosphorylation of phase-separated p62 bodies by ULK1 activates a redox-independent stress response
The article “Phosphorylation of phase-separated p62 bodies by ULK1 activates a redox-independent stress response” by Yasuhiro Fujioka, Yuko Noda, Atsushi Noda, Yukari Yamasaki, Rika Hayakawa, Kazuaki Okada, Takeshi Iwatsubo, Yoshinori Watanabe, and Nobuo N. Noda is a landmark contribution to molecular biology. Their work uncovers a novel redox-independent mechanism for NRF2 activation through ULK1-mediated phosphorylation of p62 at Ser349—a discovery that reshapes our understanding of cellular stress responses.
What sets this study apart is its exquisite use of high-speed atomic force microscopy (HS-AFM), where the NanoWorld USC‑F1.2‑k0.15 ultra-short cantilever was instrumental (https://www.nanoworld.com/Ultra-Short-Cantilevers-USC-F1.2-k0.15) . With its ultra-fast response and high-resolution capability, this probe enabled real-time visualization of dynamic molecular interactions at the nanoscale. The combination of biochemical rigor and nanotechnological precision allowed the team to capture critical conformational shifts in p62 and ULK1, validating their proposed mechanism with striking clarity.
The authors’ multidisciplinary approach, combining structural biology, live-cell imaging, and biophysics, is a masterclass in scientific innovation. This study not only deepens our knowledge of autophagy and stress signaling but also showcases how the right tools—like the USC‑F1.2‑k0.15—can push the boundaries of discovery.
EMBO J(2023)42: e113349 https://doi.org/10.15252/embj.2022113349