In mother nature, the concept of self-assembly is vital for life, as it generates much of the functionality of living cells. It also bears great synthetic potential for the formation of versatile, switchable, and functional nanostructures.*
Noncovalent interactions can be triggered by external influences, such as the change of pH, light irradiation, thermal activation, introduction of a magnetic field, moisture, or redox response. Of high interest are light-responsive systems, for example in the fields of sensors or therapy, and, thus, it is desirable to explore novel concepts toward light-triggerable self-assembly.*
In the article “Photoresponsive Photoacid-Macroion Nano-Assemblies” Alexander Zika, Sarah Bernhardt and Franziska Gröhn present light-responsive nano-assemblies with light-switchable size based on photoacids.*
Anionic disulfonated napthol derivates and cationic dendrimer macroions are used as building blocks for electrostatic self-assembly. Nanoparticles are already formed under the exclusion of light as a result of electrostatic interactions. Upon photoexcitation, an excited-state dissociation of the photoacidic hydroxyl group takes place, which leads to a more highly charged linker molecule and, subsequently, to a change in size and structure of the nano-assemblies. The effects of the charge ratio and the concentration on the stability have been examined with absorption spectroscopy and -potential measurements.*
The influence of the chemical structure of three isomeric photoacids on the size and shape of the nanoscale aggregates has been studied by dynamic light scattering and atomic force microscopy, revealing a direct correlation of the strength of the photoacid with the changes of the assemblies upon irradiation.*
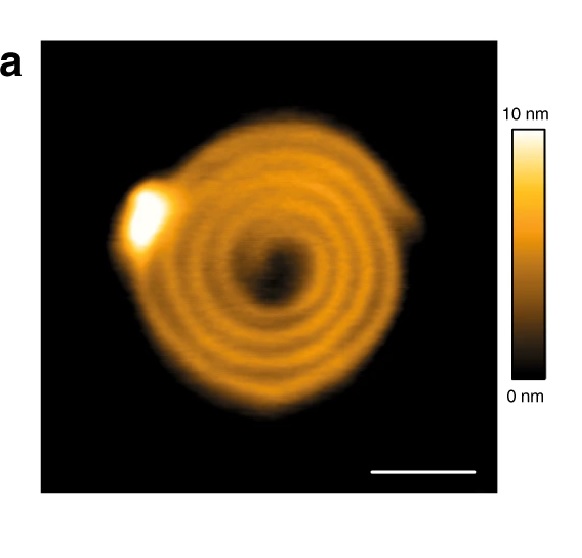
NanoWorld Ultra-Short AFM Cantilevers of the USC-F0.3-k0.3 type ( typical force constant 0.3 N/m ) were operated in tapping mode for the Atomic Force Microscopy (AFM) images presented in the article.*

Assembly formation and photoresponse of the dendrimer–photoacid system at a charge ratio of r = 0.25: (a) AFM height images before (right) and after (left) irradiation. Please refer to the full article for the full figure https://www.mdpi.com/2073-4360/12/8/1746
*Alexander Zika, Sarah Bernhardt and Franziska Gröhn
Photoresponsive Photoacid-Macroion Nano-Assemblies
Polymers 2020, 12, 1746
DOI: 10.3390/polym12081746
Please follow this external link to read the full article: https://www.mdpi.com/2073-4360/12/8/1746
Open Access : The article “Photoresponsive Photoacid-Macroion Nano-Assemblies” by Alexander Zika, Sarah Bernhardt and Franziska Gröhn is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.