In the article “Multiswitchable photoacid–hydroxyflavylium–polyelectrolyte nano-assemblies” Alexander Zika and Franziska Gröhn describe the development of a novel reversible multi-switchable system consisting of a cationic polyelectrolyte, a hydroxyflavylium molecule (Flavy), and a photoacid.*
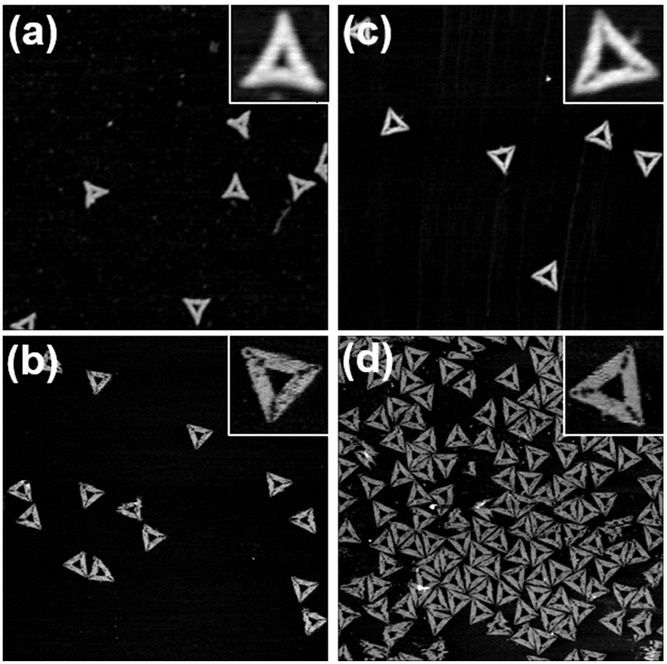
Ternary assemblies with sizes in the hundred-to-few hundred nanometers range in aqueous solution exhibit a multi-addressable size and shape.*
The concept exploits the unique property of the photoacid to form a more highly charged molecule and to switch the Flavy molecule in the same step when excited by light irradiation.*
Due to the network of possible reactions of Flavy, self-assembly can be accessed and triggered in a number of ways.*
While their study focused on the first proof of concept and the relation of molecular and nanoscale switching, a deeper understanding of the molecular binding effects may be considered in future studies.*
The type of the photoacid-based assembly presented in the article bears potential, for example, for delivery where the assembly property changes may provide a desirable transformable platform for tunable and smart transport.*
Atomic force microscopy (AFM) with NanoWorld Ultra-short Cantilevers USC-F0.3-k0.3 in tapping mode was used to investigate the structure.*

*Alexander Zika and Franziska Gröhn
Multiswitchable photoacid–hydroxyflavylium–polyelectrolyte nano-assemblies
Beilstein J. Org. Chem. 2021, 17, 166–185.
DOI: https://doi.org/10.3762/bjoc.17.17
Please follow this external link to read the full article: https://www.beilstein-journals.org/bjoc/articles/17/17
Open Access : The article “Multiswitchable photoacid–hydroxyflavylium–polyelectrolyte nano-assemblies” by Alexander Zika and Franziska Gröhn is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.